Supercharged T-cells
T-cells are an important component of the human immune defense, but their capacity to destroy cancer cells can be vastly improved through genetic engineering. In adoptive immunotherapy a patient’s own T-cells are collected and modified. The T-cells are given a new synthetic receptor which binds to antigens on cancer cells. These new T cells are called chimeric antigen receptor T-cells (CAR T). The CAR T-cells are then replicated in a laboratory then returned to a patient to attack the hematological cancer.
Not all good things last forever
Although many patients respond successfully to CAR T immunotherapy, unfortunately for others the disease can return. Relapse may occur because the CAR T-cells do not persist long enough to eliminate all tumor cells. Alternatively, it may reoccur because tumor clones have fewer antigens for the synthetic receptor to bind to, thus reducing the effectiveness of the CAR T-cells.
Double the luck
The research team suspected they could decrease the incidence of relapse by adding an additional synthetic receptor to CAR T-cells. The effectiveness of CAR T-cells is strongly influenced by other receptors that are also present on the cell, either stimulatory or inhibitory. Equipped with this knowledge, the team added a second, stimulatory receptor to increase the binding potential of the CAR T-cell. The additional receptor, a chimeric costimulatory receptor, binds specifically to antigen CD38 on tumor cells.
An avid attraction
The research team found that CAR T-cells with the added receptor (CAR+CCR T cells) have a higher attraction to tumor cells and persist longer in vivo in preclinical models. The CAR+CCR T cells also increase cytokine secretion and expansion, which is essentially a call-to-act against tumor cells. Furthermore, the CAR+CCR T-cells can better detect and kill tumor variants with low antigen density in multiple myeloma and acute lymphoblastic leukemia. Lead author and PhD student at Cancer Center Amsterdam, Afroditi Katsarou explains, “the CAR+CCR-T cells enabled the eradication of antigen-low tumor clones in vivo, which were otherwise resistant to treatment with conventional CAR T-cells.” She concludes, “the results demonstrate that the combination of CAR+CCR can impressively improve the outcomes of adoptive T-cell therapy for patients with various hematological malignancies and reduce the incidence of escaping tumor variants.” The same strategy could also find application in solid tumor therapy with the choice of appropriate target antigens.
Read the full article here: https://doi.org/10.1126/scitranslmed.abh1962
For more information, contact principal investigator and senior author of the study: Maria Themeli (m.themeli@amsterdamumc.nl).
Researchers involved from Cancer Center Amsterdam: Afroditi Katsarou, Jyoti Naik, Dionysia Kefala, Georgios Kladis, Alexandros Nianias, Ruud Ruiter, Renée Poels, Kristine A. Frerichs, Niels W.C.J. van de Donk, Sonja Zweegman, Tuna Mutis, Richard W.J. Groen, Maria Themeli.
Funders for this research was provided by: Cancer Center Amsterdam Foundation, Multiple Myeloma Research Foundation Immunotherapy Network of Excellence grant, the Swedish Research Council, National Institutes of Health grant. In this study we collaborated with LUMICKS. They provided their zMovi Cell Avidity analyzer to perform measurements of binding avidity.
Text by Lynita Howie.
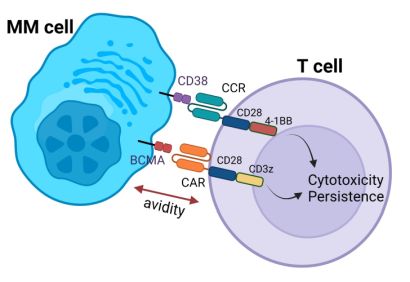 A double hit CAR T-cell displaying chimeric antigen and costimulatory receptors.
A double hit CAR T-cell displaying chimeric antigen and costimulatory receptors.

