Classical Hodgkin lymphoma (cHL) is a blood-cell cancer originating from lymphocytes and predominantly affects individuals in the age range of 20 to 40 years. In the Netherlands, approximately 430 people are diagnosed with cHL annually. The first line treatment consisting of a combination of chemo- and radiotherapy is generally effective, resulting in complete remission frequencies that approach over 80%. Nevertheless, in some patients, the disease is unresponsive to therapy or returns after an initial successful treatment.
In these refractory or relapsed patients with cHL, the immune checkpoint inhibitor anti-PD-1 has proven effective and is being rapidly incorporated into therapy regiments. This inhibitor blocks a signal on tumor cells that allows them to escape the immune system and also primes the patients’ T lymphocytes to recognize and attack the cancer. However, it is not clear which patients with relapsed/refractory cHL could benefit from anti-PD-1 therapy and how disease response can be monitored during treatment.
Response monitoring via blood sampling
Published in the journal HemaSphere, scientists at Cancer Center Amsterdam determined the potential utility of blood-based biomarkers in response monitoring for PD-1-blockade treated Hodgkin lymphoma patients. First author Esther Drees: “We collected blood samples of four Hodgkin lymphoma patients during PD-1 treatment. Extensive experimental analysis was performed on available tissue biopsies and sequential blood samples. This reflects the real-life complexity of Hodgkin lymphoma patients treated with PD-1 blockade and provides important insights into the applicability of tissue- and blood-based biomarkers for these patients.”
“We concluded that most of our tested blood-based biomarkers are very promising for disease detection and monitoring of anti-PD-1 responses in Hodgkin lymphoma, each with their own strengths and limitations,” says principal investigator Marit Roemer. “For the most accurate response monitoring, our results suggest a multi-analyte approach – that means using a combination of circulating tumor DNA, extracellular vesicle associated microRNAs (EV-miRNA) and soluble serum thymus and activation-related chemokine detection. These findings facilitate the identification of a combination of reliable predictive dynamic biomarkers that can be used in response monitoring of novel immunotherapeutic strategies.”
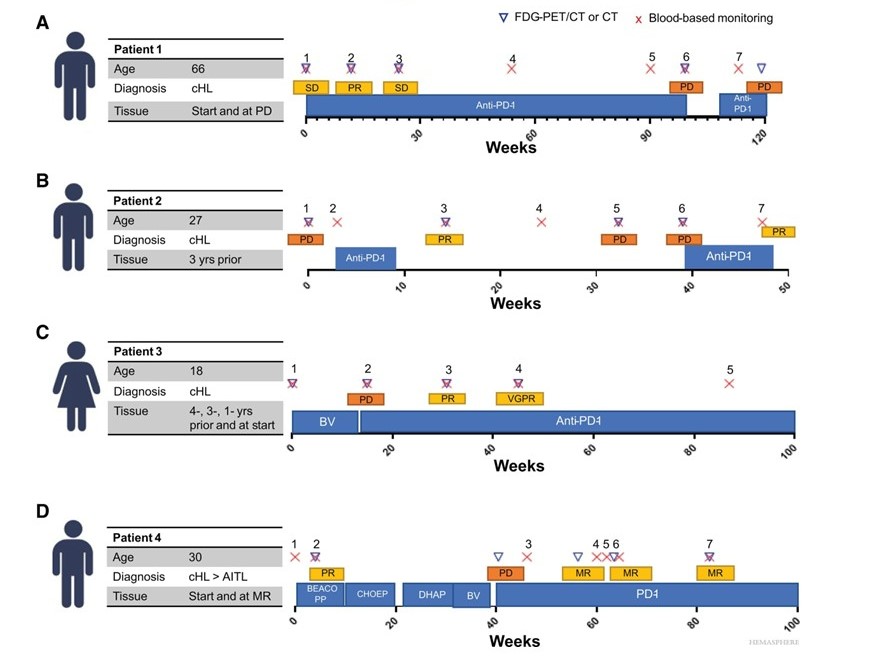
Figure. Clinical and biomarker characteristics of cHL patients that received anti-PD-1.
For more information contact Esther Drees or Marit Roemer or read the scientific publication here:
Drees, Esther E. E., et al. (2022) Blood-based Monitoring of Relapsed/Refractory Hodgkin Lymphoma Patients Predict Responses to Anti-PD-1 Treatment, HemaSphere 6 - p e749. doi: 10.1097/HS9.0000000000000749
People involved:
Esther E. E. Drees, Yvonne W. S. Jauw, Erik van Dijk, Sven Borchmann*, Sandra A. W. M. Verkuijlen, Phylicia Stathi, Nils J. Groenewegen, Nathalie J. Hijmering, Daniella R. A. I. Berry, Eric J. Meershoek*, Danielle Hoogmoed*, Anne Kwakman, Tessa J. Molenaar, Dirk M. Pegtel, Bauke Ylstra, Daphne de Jong, Josée M. Zijlstra, Margaretha G. M. Roemer.
* Not affiliated with Amsterdam UMC.
Collaborations
The researchers collaborated with Leica Biosystems/Kreatech Diagnostics B.V. for the FISH analyses. Two of the co-authors are involved in an Amsterdam UMC-based spin-off company ExBiome B.V. that focusses on development of EV-miRNA based-liquid biopsies. Internationally, we collaborated with the German Hodgkin Study Group in Cologne for the analyses of circulating tumor DNA.
Funding
The blood-based biomarker work was funded by the Dutch Cancer Society (KWF-5510), Cancer Center Amsterdam Foundation (CCA-2013), the Technology Foundation STW (STW Perspective CANCER-ID) grants awarded to D.M. Pegtel. This publication is part of the Veni project of M.G.M. Roemer with project number 101737 which is (partly) financed by the Dutch Research Council (NWO).