It has been known for decades that proteins involved in the generation of human reproductive cells are often aberrantly turned on in a myriad of cancer subtypes. But why? Dr. Gerben Vader and his team aim to uncover the role of some of these proteins in cancer development and progression in pursuit of new therapeutic opportunities.
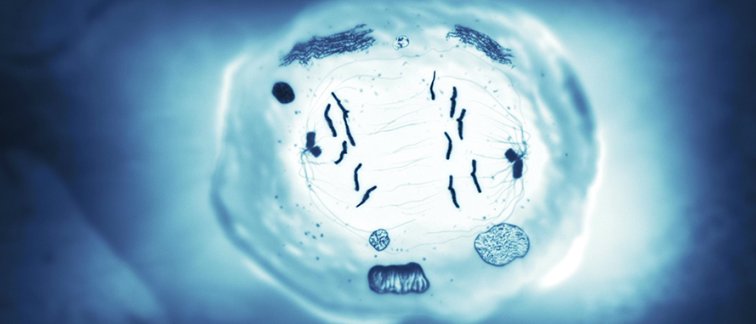
During human sexual reproduction, 23 unique chromosomes in the female egg pair up with 23 single chromosomes from the male sperm to initiate new life as a zygote with a complete set of 46 human chromosomes.
A specific cell division mechanism called ‘meiosis’ is central in the development of eggs and sperm. During meiosis, the chromosomes inherited from both parents first pair up, and swap genetic information. This so-called ‘crossing over’ results in a new combination of genetic information. This unique behavior, combined with several other processes, allows the formation of sperm and egg with only 23 chromosomes. Considering these special biologic processes, it is not surprising that a set of proteins orchestrating these events is normally needed only during meiosis.
Misplaced expression of meiosis-specific genes in cancerous cells
Regular mitotic cell division is an important process that ensures growth and maintenance of a healthy body. However, when errors occur during cell division, genomic instability and cancer can develop. While mitosis and meiosis involve several overlapping biological processes, such as DNA replication and distribution of chromosomes over daughter cells, expression of meiosis specific-genes during mitosis could potentially be harmful.

Zooming in on ‘HORMA’ signaling
In a research project funded by the Dutch Cancer Society, Vader’s research team will investigate in detail how some meiosis-specific proteins disrupt normal cell division and how that can ultimately contribute to the development of cancer. The work will focus on a family of proteins referred to as ‘HORMA proteins’ of which some normally operate during meiosis to ensure proper chromosome segregation and genomic integrity. The latest techniques in cell biology, genetics, and molecular biology will be applied to: i) understand how HORMA signaling is deregulated in cancer, and how meiosis-specific HORMA proteins contribute to such deregulation, ii) reveal the consequences of such deregulation, and iii) investigate specific vulnerabilities present in cancers that show disturbed HORMA signaling.
Dr. Gerben Vader: “The failure to maintain a stable genome is a characteristic of many cancers. Understanding the molecular processes and disruptions that feed this instability is therefore a key goal.”
PhD position genomic instability in cancer
Are you interested in discovering new phenomena that drive cancer? Funded by the Dutch Cancer Society, the Vader lab is looking to team up with a passionate graduate candidate with a Master's degree in molecular biology, biomolecular sciences, biochemistry, biology, or medical biology (or a similar field of study) with a strong interest in molecular mechanisms of genome stability and cancer.
Check this link for more information (In Dutch) and submit your application before September 4, 2023.
For more information contact Dr. Gerben Vader.
Funding provided by the Dutch Cancer Society.

This article was created for Cancer Center Amsterdam.