MRI uses strong magnetic fields to create detailed images of tumors and their surrounding tissues. This imaging technique, along with X-ray and CT-based technologies, is used in the treatment planning phase to define target tumor volumes and vital neighboring tissues. During treatment, on-board imaging guides the precise delivery of ionizing radiation to the tumors while preserving healthy neighboring tissues. Accurate delineation of the target and surrounding tissues is crucial to minimize the toxicity of radiation therapy. Imaging is also an important tool for assessing the response to treatment and real-time adaptive treatment.
25-year outlook
An international assembly of scientists, clinicians, and clinical physicists met virtually to discuss the long-term opportunities and challenges of the expanding field of MRI research and published their views recently in Magnetic Resonance in Medicine.
“MRI, with its excellent soft tissue contrast and capacity to probe functional tissue properties, holds significant untapped potential to improve radiation therapy,” says senior author and assistant professor Oliver Gurney-Champion.
In the next 25 years we expect to see:
- The superior soft-tissue visualization in comparison to computed tomography (CT) will improve tumor delineation and dosing, allowing for reduced uncertainty in target localization. This will enable more accurate treatments with less side effects as the dose is delivered more precisely.
- The recent introduction of combined radiotherapy treatment systems with MRIs will allow for concurrent imaging and tracking motion during treatment for MRI-guided radiation therapy.
- The development and availability of quantitative MRI (qMRI) techniques will add useful contrasts. Furthermore, it will be used to identify high-risk regions of the target that would benefit from dose boosts and low-risk areas where we can decrease dose and spare vital healthy neighboring tissue.
- Detecting treatment response with MRI will continue to evolve thanks to the potential of MRI to quantify the sensitivity of the tumor to radiation therapy. This information will be used to personalize dose-adaptation, for example by increasing dose in high-risk regions while lowering dose in already responding tumor regions.
- Artificial intelligence (AI) will play a major role in enabling these developments.
“Ultimately, we believe that, in 25 years, radiotherapy may consist of a single visit, during which diagnosis, personalized dose determination, AI-guided treatment planning, and treatment delivery while tracking the tumor, are all performed on a single MR-Linac system,” says Dr. Gurney-Champion
MRI at Amsterdam UMC
Amsterdam UMC is well-prepared for the future of MRI in radiotherapy and has already been using MRI in radiotherapy for almost a decade.
“Amsterdam UMC was the first in Europe to treat patients with an integrated MRI in a radiotherapy treatment system and we have been treating patients for over 5 years now,” says Dr. Gurney-Champion. MRI research at Cancer Center Amsterdam includes brachytherapy, imaging respiratory and intestinal motion, breathing control strategies designed to reduce this motion, and quantitative MRI for treatment monitoring.

For more information email Dr. Oliver Gurney-Champion, or read the review: Goodburn, RJ, et al. (2022) The future of MRI in radiation therapy: Challenges and opportunities for the MR community. Magn. Reason. Med. 88: 2592- 2608. https://doi.org/10.1002/mrm.29450
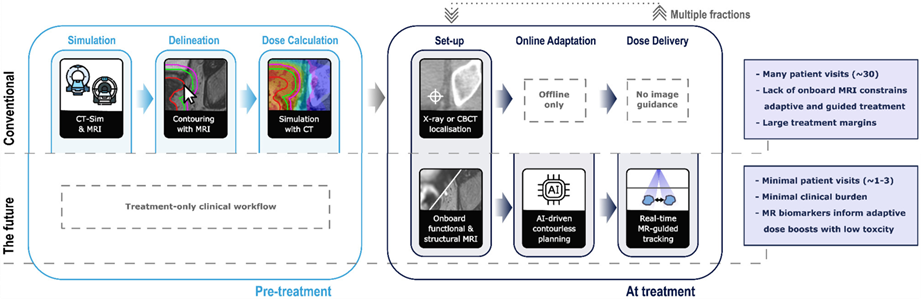
Figure. The role of imaging for radiation therapy (RT) in conventional, state-of-the-art, and future workflows.
People involved:
Rosie J. Goodburn,
Marielle E. P. Philippens,
Thierry L. Lefebvre,
Aly Khalifa,
Tom Bruijnen,
Joshua N. Freedman,
David E. J. Waddington,
Eyesha Younus,
Eric Aliotta,
Gabriele Meliadò,
Teo Stanescu,
Wajiha Bano,
Ali Fatemi-Ardekani,
Andreas Wetscherek,
Uwe Oelfke,
Nico van den Berg,
Ralph P. Mason,
Petra J. van Houdt,
James M. Balter,
Oliver J. Gurney-Champion
Funders involved:
Cancer Institute NSW, Grant/Award Number: ECF/1015; Cancer Research UK, Grant/Award Number: C33589/A28284; KWF Kankerbestrijding, Grant/Award Number: KWF-UVA 2021-13785; National Institutes of Health, Grant/Award Number: P30 CA142543
-
Text by Laura Roy and Oliver Gurney-Champion.
This article was created for Cancer Center Amsterdam.