Immune checkpoint inhibitors (ICI) are drugs that block signals on immune or tumor cells which suppress activation of immune cells. Cancer treatments with PD-1 and PD-L1 ICIs have provided significant gains in therapy effectiveness, including for patients with head and neck, or lung tumors.
However, while these patients may respond well to immunotherapy initially, secondary resistance can develop as time progresses. One of the proposed underlying mechanisms of ICI resistance is upregulation of another inhibitory checkpoint, LAG-3 (lymphocyte activation gene-3, or CD223) in the tumor microenvironment.
Visualizing a potential biomarker in humans – a clinical trial
“LAG-3 can affect how well immunotherapy works, so we wanted to find out if we can use positron emission tomography (PET) to visualize where and how much LAG-3 was present in the tumor microenvironment. Knowing this could help predict how patients might respond to specific ICI treatments,” says first author and PhD candidate Iris Miedema.
The researchers performed PET-imaging of LAG-3 using a 89Zr-labelled antibody (BI 754111) in patients who have progressed after previously successful immunotherapy treatment with PD(L)-1 ICIs. The PET signals they observed from the radiolabeled LAG-3 antibody were clearly visible and target specific, two important requirements for a potential predictive imaging biomarker.
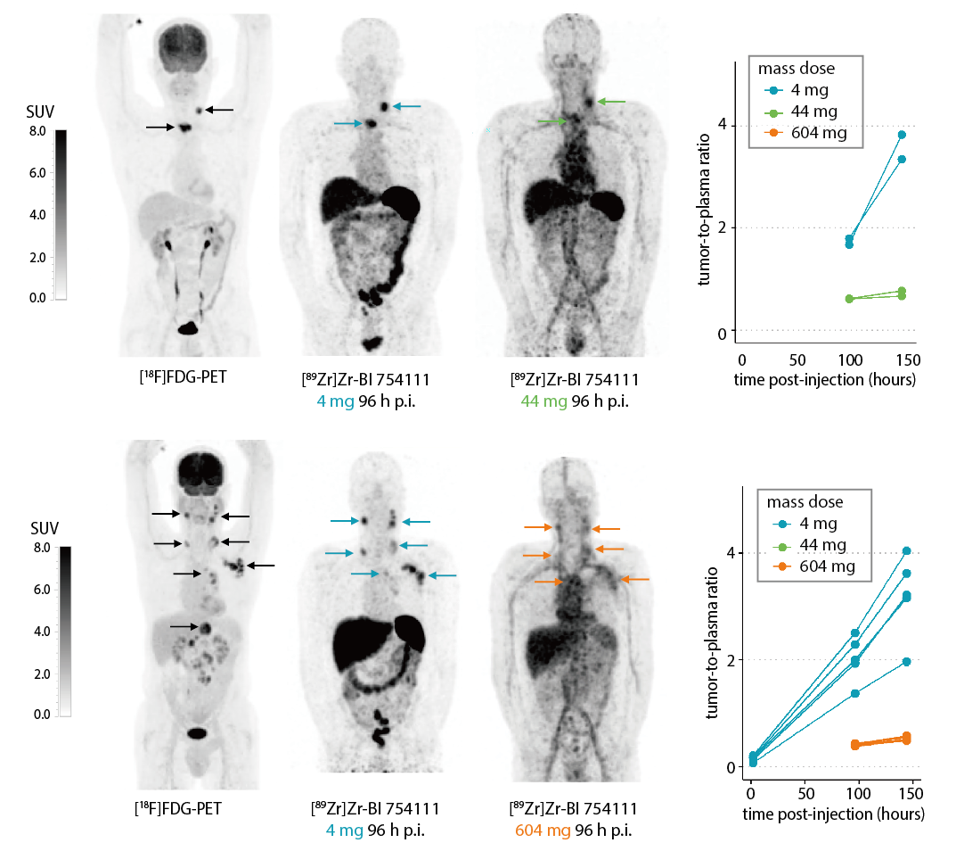
Figure. Maximum intensity projections of FDG-PET and LAG-3 PET ([89Zr]Zr-BI 754111) scans with three different mass doses of BI 754111 (4, 44, 604 mg) of one head and neck cancer patient (upper panel) and one lung cancer patient (lower panel). Tumor-to-plasma ratios are shown on the right.
Outlook
“This was the first time that PET imaging of LAG-3 was successfully performed in humans. RNA sequencing results from corresponding tumor biopsies seem to substantiate the PET-imaging results, but more research is needed in a larger patient group to confirm these findings. Still, these initial in-human imaging data clearly reveal potential of developing LAG-3 detection as a biomarker to predict patient response to specific checkpoint inhibitors,” Iris concludes.
For more information, contact Iris Miedema or read the publication:
Miedema, I.H., Huisman, M.C., Zwezerijnen, G.J. et al. 89Zr-immuno-PET using the anti-LAG-3 tracer [89Zr]Zr-BI 754111: demonstrating target specific binding in NSCLC and HNSCC. Eur J Nucl Med Mol Imaging (2023). https://doi.org/10.1007/s00259-023-06164-w
Clinical trial registration: NCT03780725.
Researchers involved at Amsterdam UMC
Iris H.C. Miedema,
Marc C. Huisman,
Gerben J.C. Zwezerijnen,
Danielle J. Vugts,
Guus A.M.S. van Dongen,
Tanja D. de Gruijl,
C. Willemien Menke-van der Houven van Oordt
Idris Bahce
Funding
This research was funded by Boehringher Ingelheim.
This article was created for Cancer Center Amsterdam.
© 2023 NHBC– All rights reserved.

