Vulvar cancer (labia cancer) is a rare type of cancer that is increasing in the Netherlands. There are 2 pathways that lead to this cancer: 1) the human papillomavirus (HPV) pathway, in which infection with HPV can lead to high-grade squamous intraepithelial lesions, the HPV-related precursor of cancer, and 2) the HPV- independent pathway, usually involving a chronic inflammatory skin disease that can lead to a HPV-independent pre-cancerous lesions.
In both pathways, vulvar cancer arises from these pre-cancerous lesions. However, not all precursor lesions develop into cancer. For example, the cancer risk for lesions caused by HPV is relatively low (10%), while women with HPV-independent neoplasia have a very high cancer risk (60%).
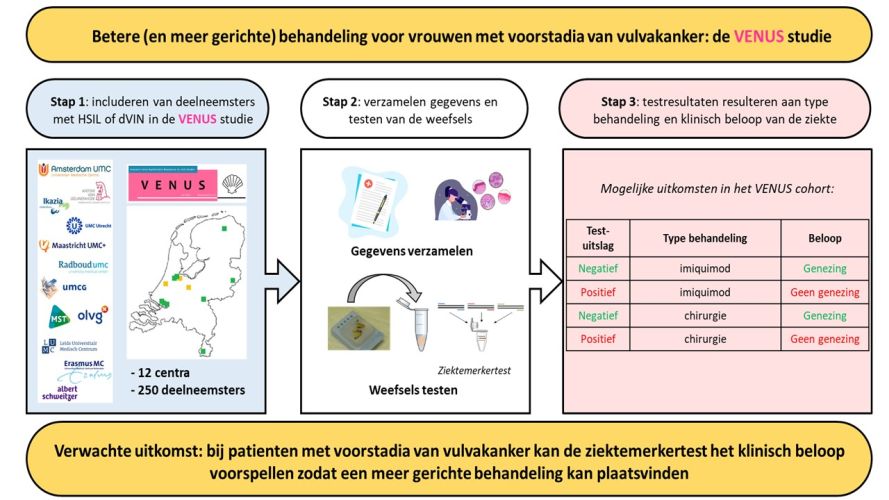
The treatment of pre-cancerous lesions usually consists of surgical removal of the lesion or treatment with a or treatment with an immune response modifier. These treatments are very taxing and (multiple) surgical procedures can be very disfiguring. Currently, the success of the various treatments cannot be accurately predicted, and abnormalities often reappear over time (up to 50%). The long-term and repeated treatments associated with this disease cause many physical and psychological discomforts, resulting in a reduced quality of life.
A better risk prediction
Dr. Maaike Bleeker, together with Prof. Renske Steenbergen, Dr. Marc van Beurden, and Prof. Hans Berkhof, are conducting a study called the Vulvar Intra-Epithelial Neoplasia in sitU Study (VENUS) in collaboration with 12 specialized centers. Also supported by previous KWF grant, the study is collecting data from women with precancerous lesions to identify prognostic factors that can be used to develop and implement a disease marker test.
The main results of the first phase of the study are expected to be published by the end of this year. In the next phase, the project leaders will use an extensive (inter)national network of clinical partners to investigate the implementation of the disease marker test.

We expect this study to show that the disease marker test we developed can better predict individual cancer risk and treatment success. This would result in a better - more targeted - choice of treatment for women with precancerous vulvar lesions. That would be an important step forward for patients and their practitioners.
For more information, contact Maaike Bleeker.
Text by Laura Roy.

