Every year 1 to 1.5 million people around the world are infected with hepatitis C with 300,000 dying from its direct consequences. “The virus causes a nasty and insidious disease,” says postdoctoral researcher Kwinten Sliepen. “Worldwide, an estimated 58 million people are infected with hepatitis C but due to the mild initial symptoms only 20 percent are aware of it. Even before you get seriously ill, you can carry the virus with you and pass it on. After 20 or even 30 years you can develop severe liver cirrhosis or cancer.”
In the blood
Hepatitis C spreads through blood contact. This makes it a sexually transmitted disease as well as one that can spread through blood transfusions or needles. The latter is currently fuelling a major epidemic among drug users in the US. While the use of contaminated blood in transfusions in Egypt has left the country with the highest rate of infection in the world. Thankfully, transfused blood is now routinely screened for the presence of the virus.
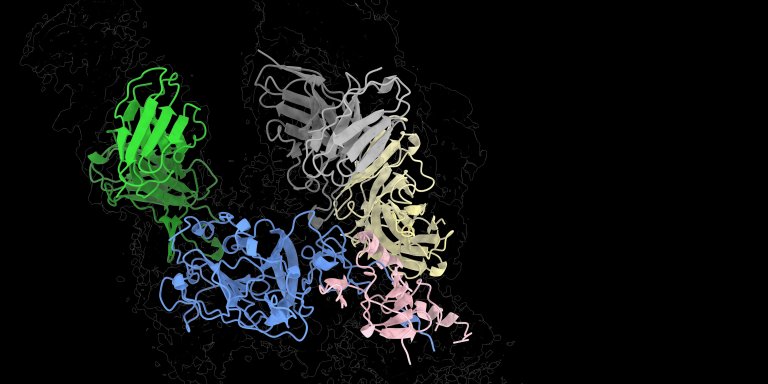
It was the buzz of the international conference on hepatitis C last summer in Ghent: 3D images of the protein E1E2 on the 'coat' of the virus. The protein is the key that hepatitis C uses to gain access to its host's liver cells. But it is not easy to pin down. The E1E2 protein changes shape continuously, so that the host's defences cannot easily grab on to it. Neither can researchers and they need to if they are to map the protein in order to develop a vaccine. In Ghent, Sliepen – finally – presented a 3D model of E1E2, after he and his colleagues had found a trick to keep the protein stable. After Ghent their findings were shared with the world in the academic journal, Science.
Tricky virus
“Hepatitis-C is a notoriously difficult virus,” says Sliepen. “There are many different hepatitis viruses and they really only have one characteristic in common: they destroy liver cells. If you look at the viruses themselves, they are very different. Vaccines against hepatitis A and B have been around for a long time, but it is still not possible to design an effective vaccine against hepatitis C.”
"At the same time, it was also a bit of a 'dormant' research field," adds Rogier Sanders, Professor of Virology at Amsterdam UMC and one of Sliepen's co-authors. “Because while there is no vaccine against hepatitis C, there are very effective antiviral medicines. Once someone is infected and becomes ill, those medicines can really cure someone. Many researchers seemed to have reconciled themselves with the availability of a cure in the absence of an effective vaccine. They seem to think 'without a vaccine we cannot prevent the disease, but we can at least cure it in more than 95 percent of cases.'"
Holy grail
Dormant until the Ghent congress, at least. The images of the enormous spaghetti of protein strands on the coat of the virus hid nothing less than the holy grail of hepatitis C research. Sliepen: “We received enormous help in our research from patients in Amsterdam, thanks to our colleague Janke Schinkel. Some had antibodies in their blood that allowed them to neutralise the virus. We have studied these antibodies down to the smallest detail. They act like hands that grab the protein E1E2 and fix it in the right shape. My colleague Alba Torrents de la Peña obtained her PhD from Amsterdam UMC and now works in California, at the Scripps Research Institute, where she can look at the structure of proteins in atomic detail. With an advanced electron microscope, she and her colleague Lisa Eshun-Wilson have made many different pictures of the E1E2 protein with the neutralizing antibodies on it. They have combined these into a three-dimensional image of the protein. We have now found the places with which we can, as it were, fix the flexible E1E2 protein.”
“This strategy is used more and more in virology,” adds Sanders. “Respiratory syncytial virus, RS virus, vaccine design has also used neutralizing antibodies to guide researchers toward the virus' Achilles’ heel.” Knowledge of the three-dimensional structure of coronavirus spike proteins has also been essential for the rapid development of the covid-19 vaccines.
Developing a vaccine
Now that the researchers literally have a picture of how an antibody can keep the protein on the coat of hepatitis C stable, the next big challenge begins: turning this knowledge into a vaccine. “In our images, we have found the places where the mobile – and therefore elusive – protein hinges, and therefore where we have to ‘build a bridge’ or ‘place a staple’ to block those hinges,” says Sliepen.
"Experience from research into HIV and the RS virus shows how such 'bridges' or 'staples' are actually formed," says Sanders. “With the amino acids cysteine or proline, you can form a connection between two parts of the protein or stabilize a certain shape. This locks the protein in the right form and gives the host's defences time to identify the protein and disarm it.”
“So, the ‘only thing’ we have to do now is to design an E1E2 protein ourselves with such a staple in it,” concludes Sliepen. “Such a mutated protein could then form the basis of a future vaccine.” Partly thanks to the mRNA vaccines that have also proved successful in the Covid-19 pandemic, Sanders and Sliepen think that there we could see a hepatitis C vaccine within 5 to 10 years.
The virus's bag of tricks
In the media, viruses are still often referred to as 'a bug'. In reality, a virus is little more than a piece of genetic material in a protein coat. A virus can do almost nothing on its own. It needs a host to multiply. A virus therefore has no interest in immediately killing its host: in a dead host it is unable to reproduce.
But the differences between Covid-19 and Hepatitis C illustrate that viruses have different strategies to exploit a host for their personal gain. “The coronavirus makes a host quite acutely ill,” says Professor of Virology Rogier Sanders. “Mutations in such a virus are relatively rare. Only if a patient's defences are compromised and a virus is allowed to hang around for a very long time can multiple mutations occur, such as in the Omicron variants that are now circulating. Within one patient you normally find no or very few different mutations.”
Hepatitis-C solved that differently. Because it can take a long time for the virus to make its host really sick, it has plenty of time to mutate. Sanders: “That makes it difficult for the immune system to tackle the virus. So, the trick is to find an unchanging piece of this protein on the coat that you can target with medicines, or better still with vaccines. With the fixed E1E2 protein now in our sights, we can finally find these targets for the hepatitis C virus.”
This text originally appeared in Dutch in Amsterdam UMC's quarterly scientific magazine Janus.
Photo: Kwinten Sliepen