Kwinten Sliepen was awarded the AII Postdoc Grant in 2021 for his research proposal titled “Structure of the hepatitis C virus E1E2 glycoprotein complex”. On the 20th of October his article was published in the prestigious scientific journal Science. Read what the AII Postdoc Grant made possible for Kwinten and why research on the E1E2 glycoprotein complex is highly relevant in the article below. Do you want to follow in Kwinten's footsteps? Application for the 2023 AII Postdoc Grant is still open until February 1st 2023, 23:59 hrs.
Kwinten Sliepen is a postdoctoral fellow at the Amsterdam Institute for Infection and Immunity, focusing on infectious diseases at the Laboratory of Experimental Virology. Together with researchers at Scripps Research in San Diego, Kwinten and colleagues at Amsterdam UMC unraveled the structure of the spike protein of the hepatitis C virus. Dr. Sliepen: ‘The structure of the spike protein has been a mystery for years. This discovery enables molecular insight into the entry of the hepatitis C virus, therefore allowing us to start designing a vaccine.'
Hepatitis C virus (HCV)
Every year, 300,000 people worldwide die from the hepatitis C virus (HCV). This virus infects liver cells causing a chronic infection. In most cases, the infection goes unnoticed, sometimes for decades, until the patient develops severe symptoms. This might be too late for antiviral treatment, because the patient has already developed liver cirrhosis and/or liver cancer. Because people do not know they carry HCV, this silent killer can continue to spread through blood-to-blood contact, such as blood transfusion, sexual contact or sharing contaminated needles.
No vaccine on the market
Unlike hepatitis A and B viruses, there is no vaccine against the hepatitis C virus (HCV). The spike on the outside of HCV, called E1E2, is the only viral protein that can generate neutralizing antibodies and therefore this protein is crucial for developing a vaccine. Until recently, however, it was unknown what E1E2 looked like.
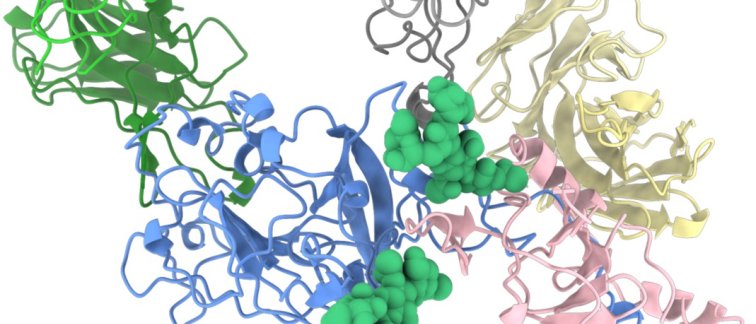
The E1E2 protein is highly mobile and flexible and therefore difficult to image. Dr. Sliepen: 'To solve this problem, we added a combination of three neutralizing antibodies that bind different parts of E1E2 to stabilize it in the correct shape. Our collaborators at Scripps Research in San Diego, Alba Torrents de la Peña, who was a former PhD student at the Laboratory of Experimental Virology in Amsterdam UMC, and Lisa Eshun-Wilson generated thousands of images of E1E2 in complex with the antibodies using electron microscopy, and these images were then reconstructed to create a 3D model of E1E2. This finally gave us an opportunity to look at the HCV spike at a high-resolution!'. In this 3D model, the team saw how E1E2 was held together by weak atomic interactions, including two sugar molecules. In addition, they could see in atomic detail how the stabilizing antibodies interacted with E1E2. Dr. Sliepen: ‘We can use this model to better understand hepatitis C virus infection and begin to design HCV vaccines and new antiviral drugs.’
What’s next?
Elucidating the structures of the Respiratory Syncytial (RS) virus and COVID-19 spikes has been paramount for the development of vaccines against RS and COVID-19. With the structural information on E1E2, researchers all over the world can get to work on developing an hepatitis C virus (HCV) vaccine. In collaboration with other laboratories the team of Dr. Sliepen continues to investigate the E1E2 protein to get a better understanding of its dynamics and design vaccine prototypes, ‘Let's just say, we have a lot of things brewing in our laboratory.’ With this high-resolution structural information the next goal would be to design soluble E1E2 vaccine candidates that remain stable without the antibodies attached. Furthermore, having atomic detail of the antibody binding regions it is possible to improve E1E2 vaccines in such a way that the immune system will more readily generate these desired antibodies.

AII Postdoc grant
Kwinten was awarded the 2021 AII Postdoc grant that made it possible for him to conduct this research project. Dr. Sliepen: ‘It was very important for me to get this grant. At the time, I had interesting clues on how to crack the “E1E2 mobility problem” which would help solving the structure of E1E2, but funding was needed to investigate this further. Thanks to the AII Postdoc grant, I was able to complete this risky project and, together with Alba and Lisa, bring it to fruition. And look where we are now: we have made a groundbreaking discovery that brings a vaccine for hepatitis C closer than ever.’
Get to know Kwinten
Conducting research is not the only thing Kwinten is into, he recently became a father of a daughter, a new kind of activity in his life in which he lovingly invests a lot of his time. In addition, Kwinten is an active member of the Amsterdam Institute of Infection and Immunity: he is a committee member of the AII postdoc committee and has recently joined the AII valorization committee. Prior to focusing on hepatitis C virus in his role as Postdoc, Kwinten conducted research on HIV-1 during his PhD. In between, he explored the world during a six-month adventure in South America.
For more information contact Dr. Kwinten Sliepen or read the scientific article in Science.
Curious to learn more about Infectious Diseases? Read our recently published articles:
Life-saving medicine for RS virus infection in babies near at hand (September 2022)
Vaginal bacteria increases risk of HIV infection (September 2022)
Corona drug for people with immune disorders elicits rapid resistance (August 2022)
Text: Esmée Vesseur
Image: E1E2 protein complex (blue = E2, pink = E1; green= sugar molecules on E1 and E2)