Despite over four decades of research, an effective vaccine for HIV remains elusive. HIV continues to be a major global health threat, affecting over 40 million people worldwide, and after multiple failed clinical trials, new and innovative approaches are urgently needed to develop a vaccine. Recently, a new vaccine strategy pioneered by Amsterdam UMC researchers has shown promise in animal models and may provide a breakthrough in the long-standing battle against the virus.
The field of HIV vaccine research is now focusing on inducing broadly neutralizing antibodies (bnAbs) as a key part of the solution. These antibodies have the ability to neutralize diverse strains of HIV by targeting the virus' envelope protein (Env). This strategy is similar to the one used in coronavirus vaccines, which aim to induce antibodies that block the virus’ spike protein from entering human cells. However, HIV’s extreme diversity makes it a more difficult target and has been one of the main reasons for the absence of an effective vaccine.
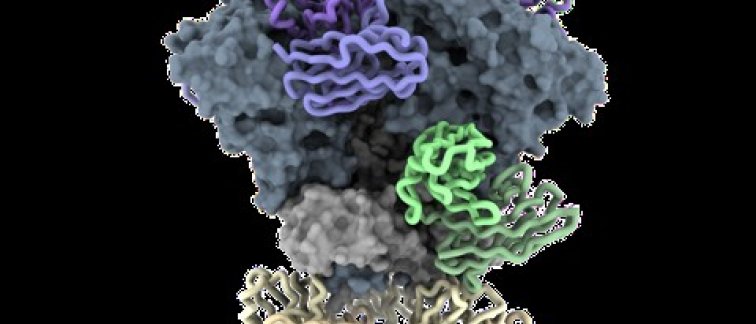
The GT1.1 vaccine
Dr. Caniels and colleagues developed a strategy to stimulate the immune system to produce these crucial bnAbs. The research centers around a novel vaccine, GT1.1, which targets "germline" precursors—the naive B cells capable of eventually becoming bnAb-producing cells. This approach aims to mimic the natural process by which some individuals develop these protective antibodies after years of evolution between the virus and the immune system.
The core question Dr. Caniels and his team aimed to answer was whether a germline-targeting strategy could activate the right immune responses to prevent HIV infection. The results, now published in Science Immunology, are promising.
Key findings
The researchers used a modified version of HIV’s Env protein, called GT1.1, designed to bind specifically to the germline precursors of bnAbs. They first tested the strategy in knock-in (KI) mouse models that simulate the human immune system. These models were able to activate the B cells with the potential to produce bnAbs, confirming that GT1.1 could successfully prime the immune system.
‘We can compare these B cells to youth football players. All famous football players started out in such a youth team, with talent scouts picking out the ones with the most potential. That is exactly what GT1.1 was designed to do: scouting B cells that have the potential to become bnAb-producing B cells’, Dr. Caniels said.
Moreover, when GT1.1 was tested in a more difficult animal model, non-human primates, it triggered antibody responses that targeted conserved regions of the HIV Env protein and successfully prevented infection with multiple HIV strains in laboratory conditions.
These antibodies were found to target critical parts of HIV’s structure—specifically the CD4 binding site (CD4bs)—demonstrating its potential to prime a broad range of bnAb precursors, even in species with slightly different immune system configurations, such as non-human primates.
Looking ahead
The findings in animal models were received well. As a result, the Gates Foundation has now funded a phase 1 clinical trial that tests the safety and promise of this vaccine in humans. This clinical trial is run at two sites in the Unites States as well as in Amsterdam. Early results indicate that GT1.1 can effectively engage the human immune system, stimulating the production of B cells capable of recognizing HIV in a manner similar to broadly neutralizing antibodies.
‘The results so far suggest that our vaccine could be a key step forward,’ said Dr. Caniels. ‘We have shown that GT1.1 can activate the immune system to generate the right kind of precursors for bnAbs, and the antibodies produced in response to the vaccine can recognize HIV in a way very similar to how naturally occurring bnAbs do. This is a huge step toward developing a protective vaccine, and we are excited to share more soon on our clinical trial.’
The research team is now analyzing the outcomes of their ongoing phase 1 clinical trial and remains hopeful that the data will support the continued development of a functional HIV vaccine. The work is supported by funding from the Bill & Melinda Gates Foundation and the NIH P01 program.
For more information contact Tom Caniels (t.g.caniels@amsterdamumc.nl) or read the scientific publication: Germline-targeting HIV vaccination induces neutralizing antibodies to the CD4 binding site.
Main researchers involved
Tom Caniels1,2, PhD, first author
Rogier Sanders1,2,3, Prof, corresponding author
John Moore3, co-leader on vaccine design
Gabriel Ozorowski4, main structural studies
Andrew Ward4, main structural studies
Facundo Batista5,6, overseeing animal immunization
1Amsterdam UMC, location AMC, University of Amsterdam, Department of Medical Microbiology and Infection Prevention, Amsterdam, the Netherlands.
2Amsterdam Institute for Infection and Immunity, Infectious Diseases, Amsterdam, the Netherlands.
3Department of Microbiology and Immunology, Weill Medical College of Cornell University, New York, NY, USA.
4Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA, USA.
5The Ragon Institute of Mass General, MIT and Harvard, Cambridge, MA, USA.
6Department of Biology, Massachusetts Institute of Technology, Cambridge, MA, USA.
Text: Esmée Vesseur
Learn more about our AI&I vaccine development research:
Peak Tick Season Hits, but Lyme Diseases Vaccine is Coming Closer (July 2024)
Prof. Rogier Sanders: Pioneering Virus Research and Shaping Vaccine Development (October 2023)
Malaria vaccine development breakthroughs from 2022 (January 2023)