Soluble FAS Ligand Enhances Suboptimal CD40L/IL-21–Mediated Human Memory B Cell Differentiation into Antibody-Secreting Cells
Saskia D. van Asten, Peter-Paul Unger, Casper Marsman, Sophie Bliss, Tineke Jorritsma, Nicole M. Thielens, S. Marieke van Ham and Robbert M. Spaapen
J Immunol July 2, 2021, ji2001390; DOI: https://doi.org/10.4049/jimmunol.2001390
Abstract
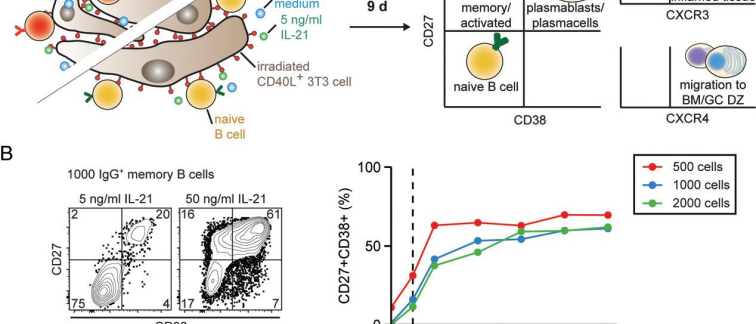
Differentiation of Ag-specific B cells into class-switched, high-affinity, Ab-secreting cells provides protection against invading pathogens but is undesired when Abs target self-tissues in autoimmunity, beneficial non–self-blood transfusion products, or therapeutic proteins. Essential T cell factors have been uncovered that regulate T cell–dependent B cell differentiation. We performed a screen using a secreted protein library to identify novel factors that promote this process and may be used to combat undesired Ab formation. We tested the differentiating capacity of 756 secreted proteins on human naive or memory B cell differentiation in a setting with suboptimal T cell help in vitro (suboptimal CD40L and IL-21). High-throughput flow cytometry screening and validation revealed that type I IFNs and soluble FAS ligand (sFASL) induce plasmablast differentiation in memory B cells. Furthermore, sFASL induces robust secretion of IgG1 and IgG4 Abs, indicative of functional plasma cell differentiation. Our data suggest a mechanistic connection between elevated sFASL levels and the induction of autoreactive Abs, providing a potential therapeutic target in autoimmunity. Indeed, the modulators identified in this secretome screen are associated with systemic lupus erythematosus and may also be relevant in other autoimmune diseases and allergy.