With the Nobel prize-winning application of CRISPR technology in human cells, it recently became possible for researchers to systematically knock-out human genes in lab-grown cells to reveal their roles in cancer relevant biological processes.
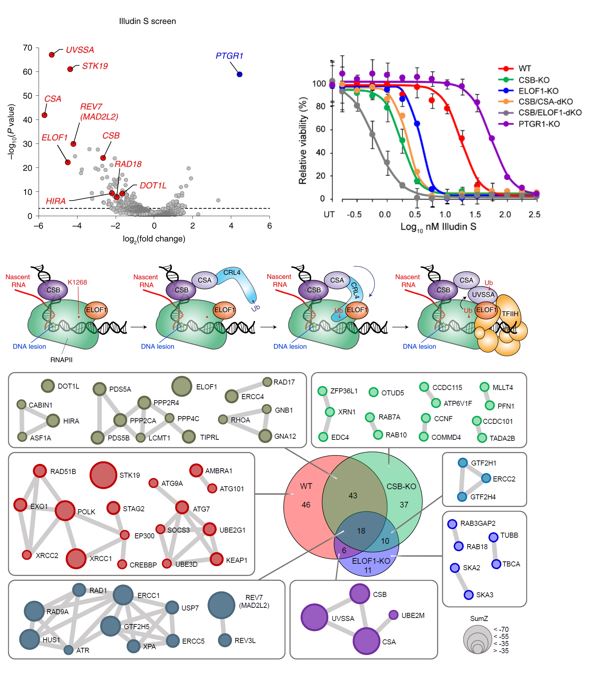
The Wolthuis lab (Oncogenetics, Department of Human Genetics) at the Cancer Center Amsterdam, implemented a new pipeline for so-called pooled lentiviral full-genome CRISPR-Cas9 knockout screening. A collection of 71,000 different CRISPR-lentiviruses was used to create a population of millions of cells, each containing a knockout of a unique human gene. Subsequently it was measured which genes are essential for cell growth and survival, before or after treatment with a specific DNA-damaging drug. Thereby the researchers were able to pinpoint the human genes and pathways involved in the underlying DNA repair pathway of this study, called Transcription-Coupled DNA Repair (TCR).
Apart from all the known TCR genes, the previously uncharacterized ELOF1 gene was identified as a top hit. Next, a wide range of experimental approaches was used, including follow-up drug screens in isogenic cell lines, to elucidate the precise function of ELOF1. The researchers discovered that ELOF1 is a novel component of the mRNA synthesis machinery (called the RNA-polymerase II complex) but also acts as an important factor in DNA repair, when the RNA-polymerase II complex encounters a transcription-blocking lesion.
ELOF1 is particularly important to help cells deal with UV and chemotherapeutic compounds, but also plays a role in controlled cell division. These findings helps in the understanding of hereditary cancer syndromes which are linked to DNA damage repair genes, as well as the effects of targeted drugs used in cancer therapy.
Link to website/publication:
Publication in Nature Cell Biology:
https://www.nature.com/articles/s41556-021-00688-9
News & views:
https://www.nature.com/articles/s41556-021-00698-7
Back-to-back publication by Geijer et al.:
https://www.nature.com/articles/s41556-021-00692-z
People involved :
(bold = Cancer Center Amsterdam/Amsterdam UMC)
Yana van der Weegen*, Klaas de Lint*, Diana van den Heuvel, Yuka Nakazawa, Tycho E. T. Mevissen, Janne J.M. van Schie, Marta San Martin Alonso, Daphne E. C. Boer, Román González-Prieto, Ishwarya V. Narayanan, Noud H. M. Klaassen, Annelotte P. Wondergem, Khashayar Roohollahi, Josephine C. Dorsman, Yuichiro Hara, Alfred C. O. Vertegaal, Job de Lange, Johannes C. Walter, Sylvie M. Noordermeer, Mats Ljungman, Tomoo Ogi, Rob M.F. Wolthuis# & Martijn S. Luijsterburg#
* Shared first authors.

