In a review published in Seminars in Cancer Biology, first author Lenka Boyd and colleagues dive into the biology of cancer-associated fibroblasts (CAFs) in the development and treatment-resistance of pancreatic ductal adenocarcinoma. The tumor microenvironment of pancreatic ductal adenocarcinoma (PDAC) is a complex matrix with a wide variety of cells like pancreatic stellate cells, fibroblasts, endothelial, and immune cells. There are also many excreted messenger molecules including cytokines, growth factors and signals packaged in extracellular vesicles.
“The tumor microenvironment has essential roles in pancreatic ductal adenocarcinoma and offers possibilities to identify new cancer biomarkers or therapeutic strategies,” explains Lenka. “However, our knowledge about it - and in particular the CAFs - is incomplete. From existing literature, we have extracted and combined crucial information about the dual roles of CAFs in PDAC pathophysiology to provide a basis for new insights and therapy development.”
Dissecting the double agents
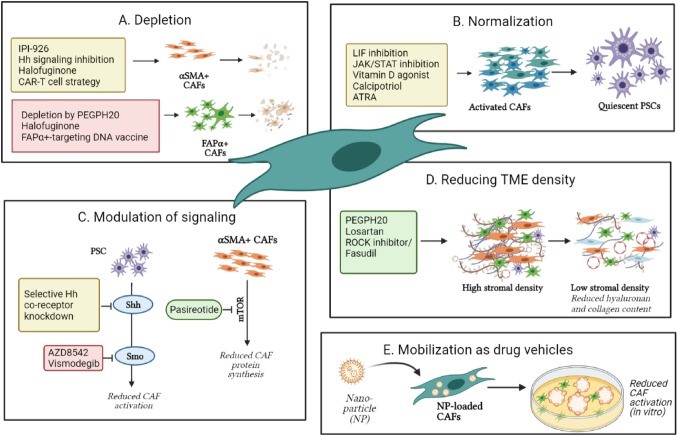
In this review, the researchers seek to clarify the intriguing heterogeneity and plasticity of CAFs, features that are key to their diverse roles in the regulation of the proliferation and differentiation of adjacent epithelial cells and the modulation of wound healing processes. “In fact, there is a great diversity of CAF functions, and these can both promote and limit the growth of tumors,” says Lenka. “For example, CAFs can encourage oncogenesis in a number of ways such as abnormal wound healing stimuli and immune suppression. But CAFs are also capable of restraining tumor growth. Therapeutic strategies aimed at depleting CAFS have produced conflicting and controversial results.”
The better understanding of the biology and dynamics of CAFs within the tumor microenvironment will allow the use of CAFS as prognostic or predictive biomarkers which could open the door to better clinical management and personalized therapeutics.
Research activities at Cancer Center Amsterdam to clarify the dual role of CAFs in PDAC are ongoing.
For more information contact Lenka Boyd or read the Scientific Review here:
Boyd, L.N.C., et al (2022) Heterogeneity and plasticity of cancer-associated fibroblasts in the pancreatic tumor microenvironment. Seminars in Cancer Biology 82, 184-196. https://doi.org/10.1016/j.semcancer.2021.03.006
Funding for this work was provided by the Bennink Foundation and Stichting Cancer Center Amsterdam
Involved researchers at Cancer Center Amsterdam:
Godefridus J. Peters
Geert Kazemier
Elisa Giovannetti
In collaboration with:
Katarina D. Andini, the Netherlands Cancer Institute