Neuropeptides (including neurothrophins like BDNF) are by far the largest group of chemical signals in the brain. Neuropeptides are secreted from dense-core vesicles (DCVs) and control many physiological functions such as brain development, synaptic plasticity, circadian rhythm, behavior and emotions. Defects in neuromodulator signaling are associated with multiple psychiatric disorders, obesity and diabetes, still, their secretory pathway is poorly understood, e.g., in comparison to neurotransmitter release from synaptic vesicles (SVs). While many excellent studies have characterized the specialized release sites for SVs, the active zones, the organizing principles for neuropeptide release from DCVs remain elusive.
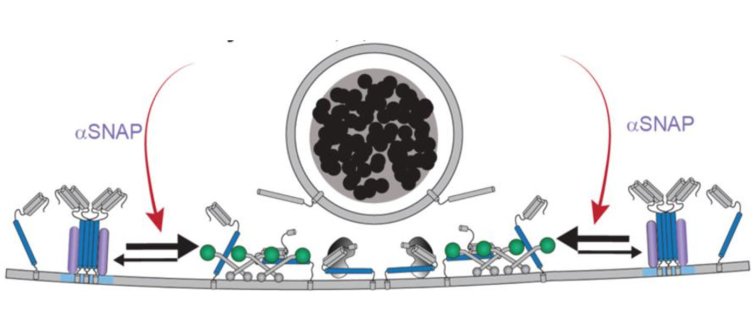
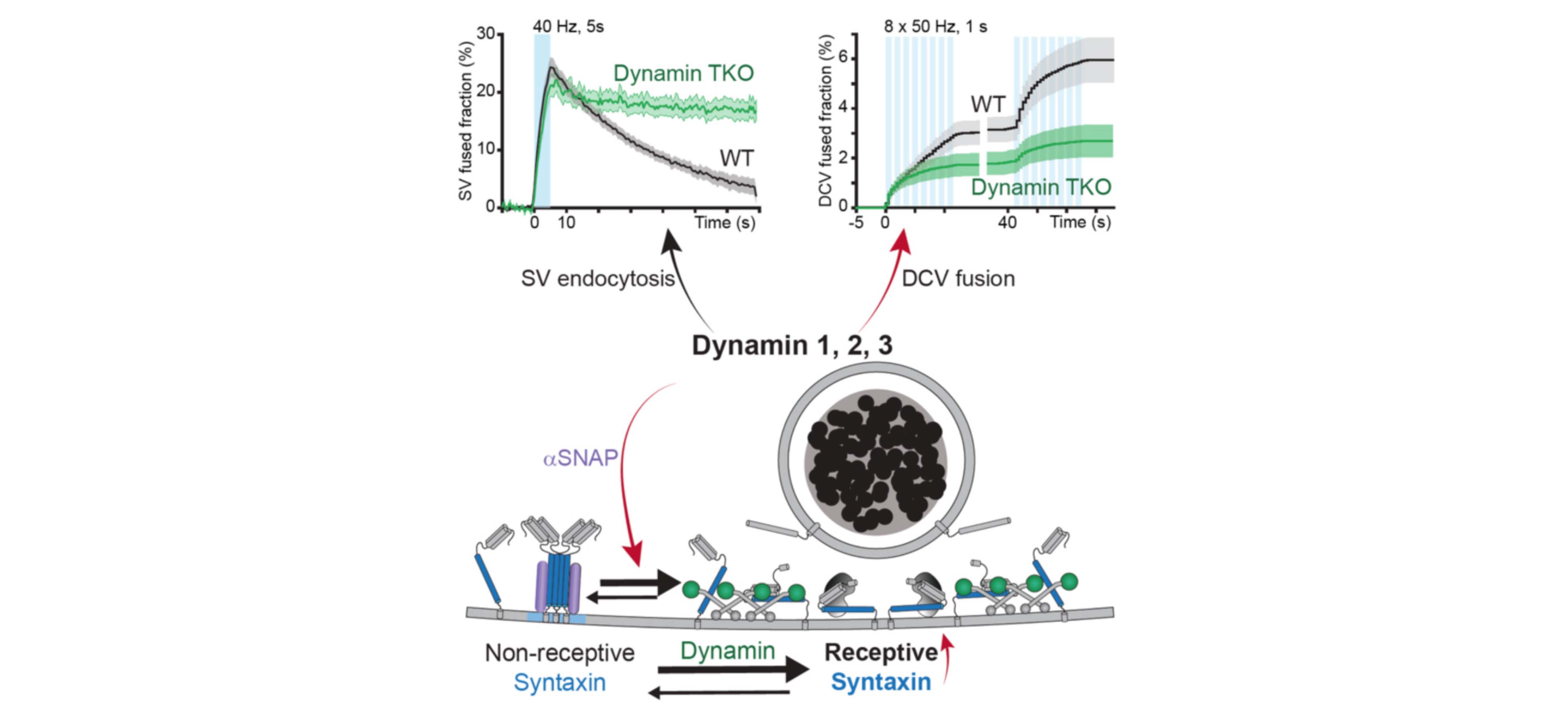
In this study, starting from the organization principles of yeast vacuolar fusion, Alessandro Moro and Anne van Nifterick identified dynamins, yeast Vps1 orthologs, as important DCV fusion site organizers in mammalian CNS neurons. Genetic or pharmacological inactivation of all three dynamins strongly impaired DCV exocytosis, while SV exocytosis remained unaffected. Wildtype dynamin restored normal exocytosis, but not GTPase-deficient or membrane-binding mutants that cause neurodevelopmental syndromes. The authors showed that, in the absence of dynamin, a significant proportion of syntaxin1 was present in inaccessible clusters that could be restored by αSNAP overexpression. Together, the data indicate that dynamin promotes DCV fusion site organization, downstream of SNARE disassembly, by regulating the equilibrium between fusogenic and non-fusogenic syntaxin-1 and promoting its availability to form trans-SNARE complexes that drive DCV exocytosis.
Read the article in Science Advances: Dynamin controls neuropeptide secretion by organizing dense-core vesicle fusion sites