Research Portal effective
On February 3, 2025, the Research Portal, an internet portal for registering, submitting and managing medical-scientific research, will be effective in the Netherlands. The Research Portal replaces ToetsingOnline, which means there will be significant changes for researchers in the registration and submission process. Detailed information can be found on the CCMO website. ToetsingOnline is now only available for completing ongoing procedures (submissions). In the course of 2025 ToetsingOnline will go offline. Until then, users will have read only access to the system.
The Research Portal will be used to submit all medical research submissions that fall under the scope of the WMO (Medical Research Involving Human Subjects Act), the Embryo Act, MDR, IVDR, as well as national non-interventional PASS studies at the request of the CBG, and the DRCFs non-WMO review framework (non-WMO research initiated and/or funded by pharmaceutical companies). The submission and evaluation of non-WMO research will continue to be processed through Research Manager at Amsterdam UMC.
This also applies to the submission and evaluation of biobank setup and use of an already existing biobank.
For clinical trials with medicinal products, submission and evaluation will continue via CTIS. Local feasibility for Amsterdam UMC remains the same and must be requested by the researcher through Research Manager.
What does this mean for you?
For medical scientific research falling under the scope of the WMO, but not subject to the CTR (medicinal products), the submission process has changed. Previously,
you submitted your research through Research Manager. However, starting February 3,
all submissions (including amendments, SAEs and progress reports) must now be submitted via the Research Portal. Local feasibility will still be processed through Research Manager.
Below, you find information on where to submit your research, which review committee is involved, and how local feasibility requests are handled for each type of research.
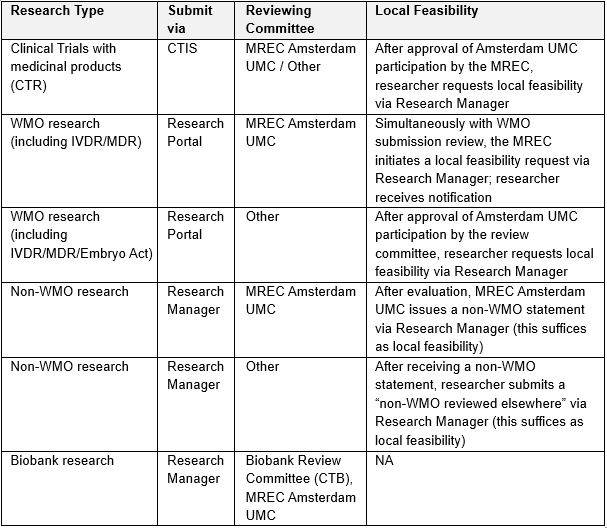
For medical scientific research under the scope of the WMO, but not subject to the CTR (medicinal products), you no longer need to request local feasibility via Research Manager yourself. After the MREC reviews the research, you will receive the local feasibility request in Research Manager along with the committee's questions.
Expansion of the use of the Site Suitability Declaration (VGO)
For all WMO research conducted in Amsterdam UMC, starting March 1, 2025, a Site Suitability Declaration (VGO) for Amsterdam UMC must be submitted with the first submission in the Research Portal. This is in line with national developments.
Until March 1, 2025, a research declaration for research conducted at Amsterdam UMC can still be submitted instead of the VGO.
Updated template submission letter
We would also like to bring to your attention that the template for the submission letter for new WMO applications for MREC review has been updated. You must use this new letter to have your application processed.
Please refer to the MREC website for further information. If you have any questions, please contact the METC Office at metc@amsterdamumc.nl.
DocuSign available for digital signing
Since January 1, 2025, everyone at Amsterdam UMC can use DocuSign for secure and easy digital signing*. DocuSign is now centrally procured. You can request access through the Service Portaal.
For departments that have already purchased envelopes, these can still be used before ordering new envelopes via the Service Portaal (one-time registration). Payment will still be processed through the department’s cost center.
An envelope costs €2.38. You can attach many documents in different formats to one envelope.
If you have not worked with DocuSign before, please read this news article and this manual on how to use DocuSign.
*For Medical Research Involving Human Subjects, there are a few important points to consider:
- DocuSign is not automatically allowed for digital signing of informed consent for WMO research. According to the WMO, there are general conditions: the procedure must be appropriate, sufficiently reliable, and confidential.
- It must be documented in the research protocol and reviewed and approved by the review committee.
- The "site signature and delegation log" is difficult to maintain properly and GCP compliant with digital signatures and will often be kept on paper.
- Please note: Documents that are originally digital can be stored digitally (e.g., protocols, manuals, IBs, etc.). Ensure that these digital documents, including those signed via DocuSign, are part of the "Trial Master File" or "Investigator Site File" and are stored in accordance with the specific study's retention period.
For questions about using DocuSign, please contact kwaliteitsborging@amsterdamumc.nl
METC Office, Quality Assurance Clinical Research, Clinical Monitoring Center

