Rik van der Kant (Vrije Universiteit Amsterdam, Amsterdam UMC) and his colleagues Larry Goldstein (University of California San Diego) and Rik Ossenkoppele (Amsterdam UMC, University of Lund Sweden) published a review in Nature Reviews Neuroscience with new insights on the Alzheimer disease (AD) pathology. In the review, they summarize how accumulation of amyloid-β (Aβ) plaques precedes tau neurofibrillary tangle in AD patients. “The correlation between Aand tau is often interpreted as causation, where Ais considered the main driver of tau pathology and neurodegeneration in AD,” says Van der Kant. In the review, the authors highlight a number of pathways that drive tau protein accumulation independently of amyloid. For example, Van der Kant and his colleagues review evidence showing that tau accumulation can be due to disturbed cholesterol metabolism, endosomal transport, microglial activation. Cholesterol for example directly regulates degradation of tau (see figure below), and is of particular interest as novel brain-cholesterol targeting drugs that have recently been discovered.
Van der Kant enjoyed working together with Rik Ossenkoppele. He says: “Working with Ossenkoppele thought me so much about the clincial and imaging aspects of AD. We hope that our interdisciplinary review can catalyze the translation of novel concepts emerging from preclinical work to novel therapies that benefit patients here at the VUmc and worldwide.”
These findings encourage others to reconsider the classical way of thinking about amyloid and tau for the onset of AD.
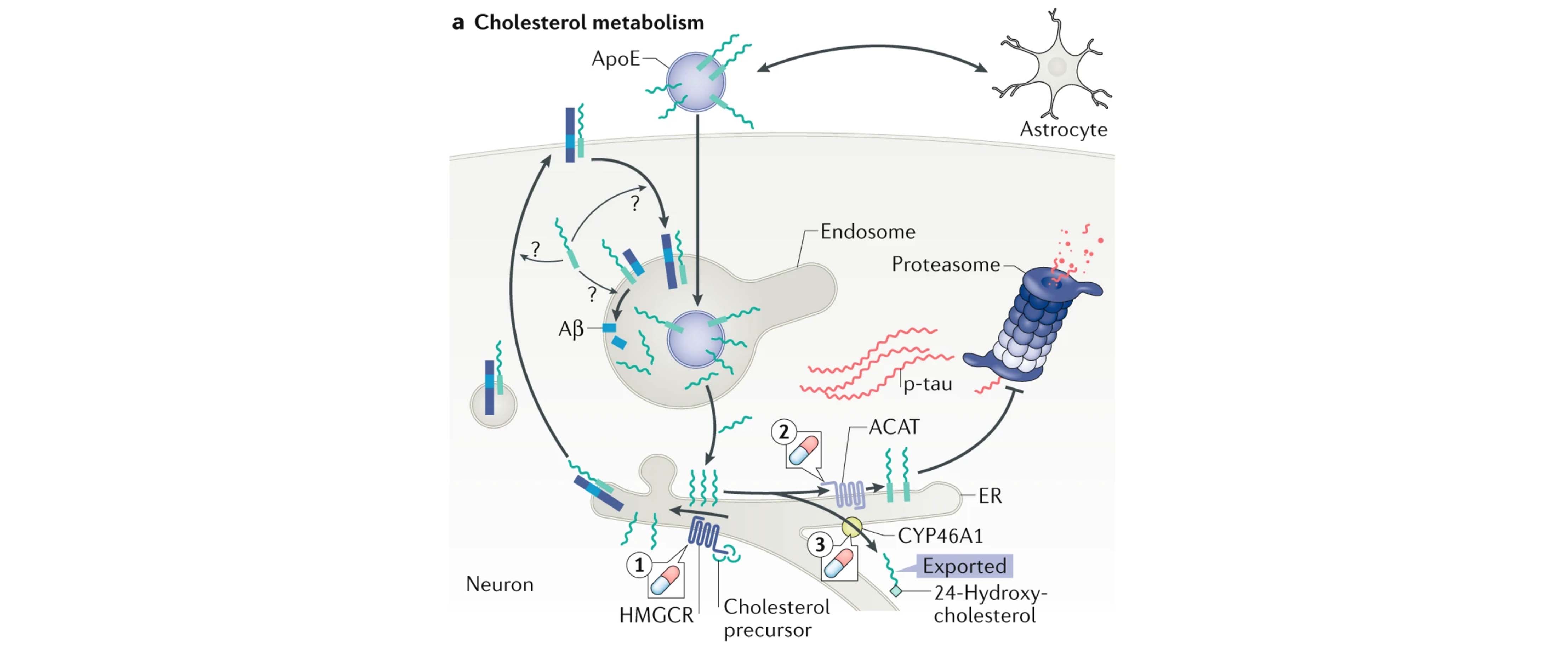 Figure from the review illustrating the cholesterol metabolism as a sporadic AD-associated pathway that act as upstream regulators of both Aβ and tau pathology.
Figure from the review illustrating the cholesterol metabolism as a sporadic AD-associated pathway that act as upstream regulators of both Aβ and tau pathology.
Read the article in Nature Reviews Neuroscience 'Amyloid-β-independent regulators of tau pathology in Alzheimer disease'.