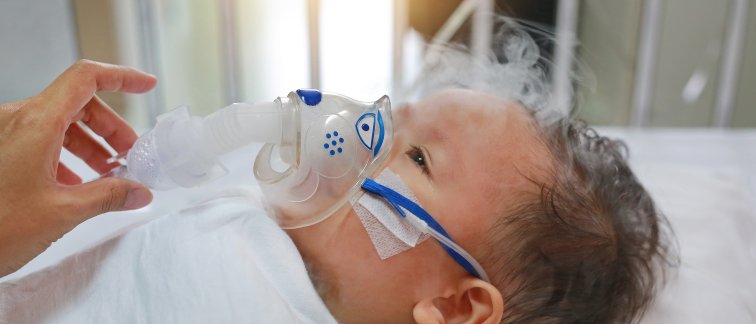
Every year, 150 to 200 babies with respiratory syncytial virus (RS virus) end up in the ICU in the Netherlands. Worldwide, the RS virus is the second leading cause of death in infants. Researchers at Amsterdam UMC discovered an antibody that protects against this life-threatening virus for newborns. Last month, the EMA issued a positive advice for this antibody drug, called Nirsevimab. One more step to go: once the European Commission has approved the drug, it can be used in the fight against the RS virus.
RS virus is a respiratory viral disease that causes symptoms such as breathing problems. It is the most common cause of inflammation of the small airways in the lungs (bronchiolitis) and pneumonia in infants. RS virus is spread when an infant comes in contact with fluid from the nose or mouth of an infected person.
Prevention of suffering and death worldwide
'The discovered antibody can help prevent babies from developing a life-threatening infection with the RS virus. Although there is sufficient ICU capacity in the Netherlands to treat this infection in newborns, an ICU admission has an enormous impact on the child, parents and family. In less affluent countries without ICU capacity, a serious infection with the RS virus in babies can even be fatal. The remedy with this antibody medicine thus prevents a lot of suffering and death worldwide,’ says Professor of Pediatric Intensive Care Job van Woensel.
Immortal B cells
Antibodies are made by so-called B cells, also known as white blood cells. These cells recognize a viral infection and respond by making antibodies. In 2007, researchers at Amsterdam UMC led by immunologist Hergen Spits discovered a new technique to make B cells immortal in a culture dish. Using this technique, his colleagues Tim Beaumont and Etsuko Yasuda isolated an antibody that inactivates the RS virus. To do so, they used the blood of a healthy adult who was not affected by the virus.
Premature infants
The license of this research was transferred to the pharmaceutical company Astra Zeneca 15 years ago. After the necessary follow-up research and some clinical trials, preliminary approval for the antibody was given by EMA, the European Medicines Agency, in September 2022. Once the European Commission gives approval, the drug will enter the market. 'We then hope that the antibody will initially be given quickly to children with the highest risk of a severe course of infection with the RS virus. These are premature babies and babies with congenital defects,' said Tim Beaumont, researcher at Amsterdam UMC. 'But healthy, full-term babies can also become very seriously ill from this virus. Ultimately, all newborns will benefit from preventive treatment with Nirsevimab.'
Curious to learn more about Infectious Diseases? Read our recently published articles:
Deadly Marburg virus surfaced in Ghana (August 2022)
Monkeypox virus detected in a child in Amsterdam: 'We don't know how he got infected' (July 2022)
Plenty of theories, but long covid remains a mystery (June 2022)