Grant Winner: Mohammed Ghiboub
Grant type: ECCO grant
Description of the grant: The European Crohn’s and Colitis Organization (ECCO) offers this ECCO grant to encourage and support early career individuals and promote innovative scientific research in the area of Inflammatory Bowel Diseases in Europe.
Grant amount: 80.000 euros
AGEM program: Gastroenterology
Other AGEM researchers involved in this project: prof. Wouter de Jonge and prof. Joep Derikx
Role of serotonin in visceral hypersensitivity
Visceral hypersensitivity (VH), a significant yet enigmatic issue in the realm of gastrointestinal (GI) disorders, profoundly affects the lives of up to 70% of Crohn’s Disease (CD) and 40% of Irritable Bowel Syndrome patients. VH manifests as a heightened sensitivity to pain or discomfort within the GI tract, leading to an exaggerated pain response to stimuli that should be relatively benign (Ceuleers et al, 2016). This condition not only severely impacts the daily quality of life of sufferers, but also presents a persistent challenge in clinical remission, where approximately 40% of CD patients continue to experience VH-induced abdominal pain. At the core of VH's intricate pathology lies serotonin, a crucial component of the gut-brain axis (Ceuleers et al, 2016). Intriguingly, emerging research points towards serotonin dysregulation as a key factor in exacerbating gut sensation and pain in conditions like CD, highlighting its significance beyond its well-known role in mood regulation (Salaga et al, 2019 and Wood, 2001). However, the molecular mechanisms of serotonin in mediating inflammatory response and VH are unclear, representing a significant gap in GI research.
Serotonin and the epigenetic code
Dr. Mohammed Ghiboub's pioneering research, supported by the prestigious ECCO grant, is set to explore new frontiers in understanding VH. A central aspect of his research proposal involves investigating the recently discovered phenomenon of histone serotonylation (H3Q5ser) – the covalent attachment of serotonin to glutamine residues at position 5 (Q5) on the histone H3 tail (figure 1) (Farrelly et al, 2019). This groundbreaking discovery may transform our understanding of serotonin's function, presenting it not only as a neurotransmitter but also as an epigenetic mark, potentially regulating chromatin architecture and gene expression. Recent findings of dr. Ghiboub et al. have been particularly exciting, revealing that increased serotonin levels were associated with strong enrichment of H3Q5ser in the intestinal mucosa, including the immune cells in active CD patients.
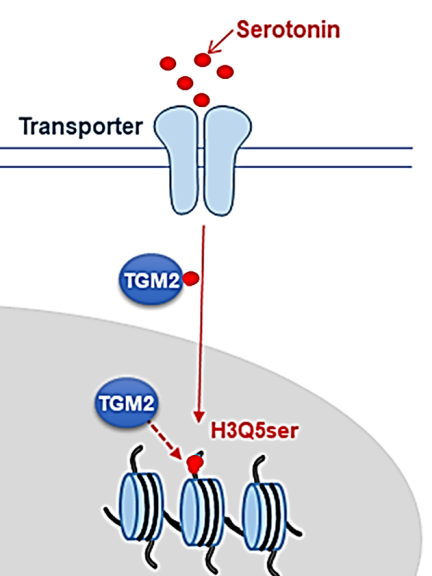
These revelations open new and promising avenues for research into the effects of serotonin-mediated H3Q5ser on gene expression, particularly genes linked to inflammation and VH. By employing a suite of advanced scientific approaches and techniques such as gene mutation, CyTOF analysis, ChIP-seq, ATAC-seq, and RNA-seq, his study will examine how serotonin and H3Q5ser are distributed across various cell types, and what their subsequent effects are on chromatin remodeling and gene expression in immune cells and sensory enteric neurons.
Outlook
Focusing specifically on pathways associated with inflammatory response and VH/pain sensation, this research has the potential to unlock a deeper understanding of the underlying mechanisms of VH. The insights gleaned from this study could pave the way for the development of novel therapeutic targets and biomarkers, revolutionizing the approach to treating VH and enhancing the quality of life for patients suffering from GI disorders. With its innovative approach and potential for significant breakthroughs, Dr. Ghiboub’s work stands as a beacon of hope in the ongoing quest to unravel the mysteries of GI health and patient care.


