Computational techniques are increasingly used to translate preclinical concepts to clinical applications in psychiatric disorders. For instance, perturbations to excitation (E) and inhibition (I) balance affecting cognition have been suggested in many disorders but methods to measure E/I ratios in human brain networks have been lacking. To tackle this challenge, the group of Klaus Linkenkaer-Hansen has been long focusing on “criticality” as a strong theory-driven translational framework. The assumption is that a network poised at the critical point exhibits optimal information processing capabilities and that this state requires E/I balance. In Klaus’ group, Richard Hardstone has used criticality to develop a method to quantify E/I ratio (fE/I) from neuronal oscillations using their computational model of critical oscillations (CROS). Child Psychiatrist Hilgo Bruining and his team recently joined Amsterdam UMC following intensifying collaboration with the group of Klaus to optimize clinical translation of this solid framework.
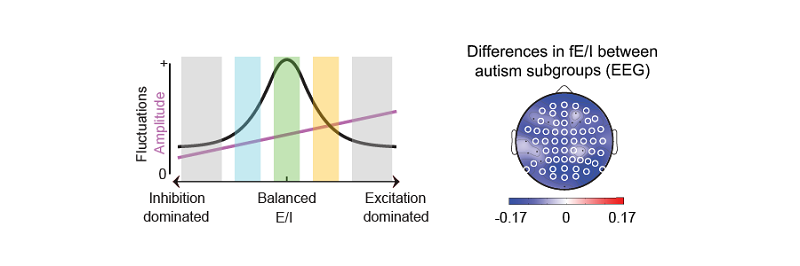
Klaus Linkenkaer-Hansen: “The concept of E/I balance has been around for decades without a consensus on how to define it. Our computational model allowed us to formally define a functional E/I ratio and study its relationship to excitatory and inhibitory connectivity and synaptic currents in-depth. We then applied the algorithm to the EEG of healthy adults and showed that fE/I is very close to 1.00 at the population level. We also show that fE/I is decreased through GABAergic enforcement, suggesting it could be used to study pharmaco-dynamics of E/I-regulating drugs or to study E/I dysregulation in humans. It has been challenging to align computational modeling with new method development and testing in three large empirical data sets. Many people contributed to the work, which is also reflected in the long author list. Everyone played an important role!”
Hilgo Bruining: “As soon as possible, we want to get beyond one-size-fits-all treatment of neurodevelopmental disorders. We know from animal models that both increased and decreased E/I ratios can be involved in pathogenesis, a finding that is largely neglected in clinical practice. We tested the algorithm on EEGs of more than 100 unmedicated children with autism spectrum disorder and in line with preclinical evidence observed a larger variance of fE/I in autism cohort. Importantly, with the help of PhD student Erika Juarez, we found that especially children without epileptiform abnormalities, fE/I was elevated. We are now finalizing the analysis of an unique EEG accompanied medication trial to show that fE/I values can be used to guide more personalized treatment allocation, which would be a major breakthrough in the field of neurodevelopmental disorders”.
Because of the clinical potential, the researchers have filed a patent on the method to protect the IP for commercial use. However, the method is freely available for academic research and we also welcome collaborations. Only a few weeks back, VUmc signed a formal collaboration with Roche to further validate the technology under the supervision of Bruining and Linkenkaer-Hansen.
Read about the study in Scientific Reports Measurement of excitation-inhibition ratio in autism spectrum disorder using critical brain dynamics