Understanding the biological diversity associated with AD is essential for the development of efficient diagnostic tools and disease-modifying therapies. Cerebrospinal fluid (CSF) reflects the antemortem biochemical alterations occurring in the brain and can thus provide the pathobiological fingerprint of different neurodegenerative disorders.
Reflection of the multifactorial nature of Alzheimer’s disease
In this study, 665 proteins in 797 CSF samples were measured from patients along the AD continuum, non-AD dementias, and cognitively unimpaired controls using proximity ligation-based immunoassays. Compared to the last two groups, more than 100 CSF dysregulated proteins along the AD continuum were identified. This reflected the complex and multifactorial biology of AD. Proteins dysregulated in patients with mild cognitive impairment with abnormal amyloid were primarily related to protein catabolism, energy metabolism and oxidative stress. Yet the dysregulated proteins AD dementia were related to different processes, namely to cell remodelling, vascular function and immune system.
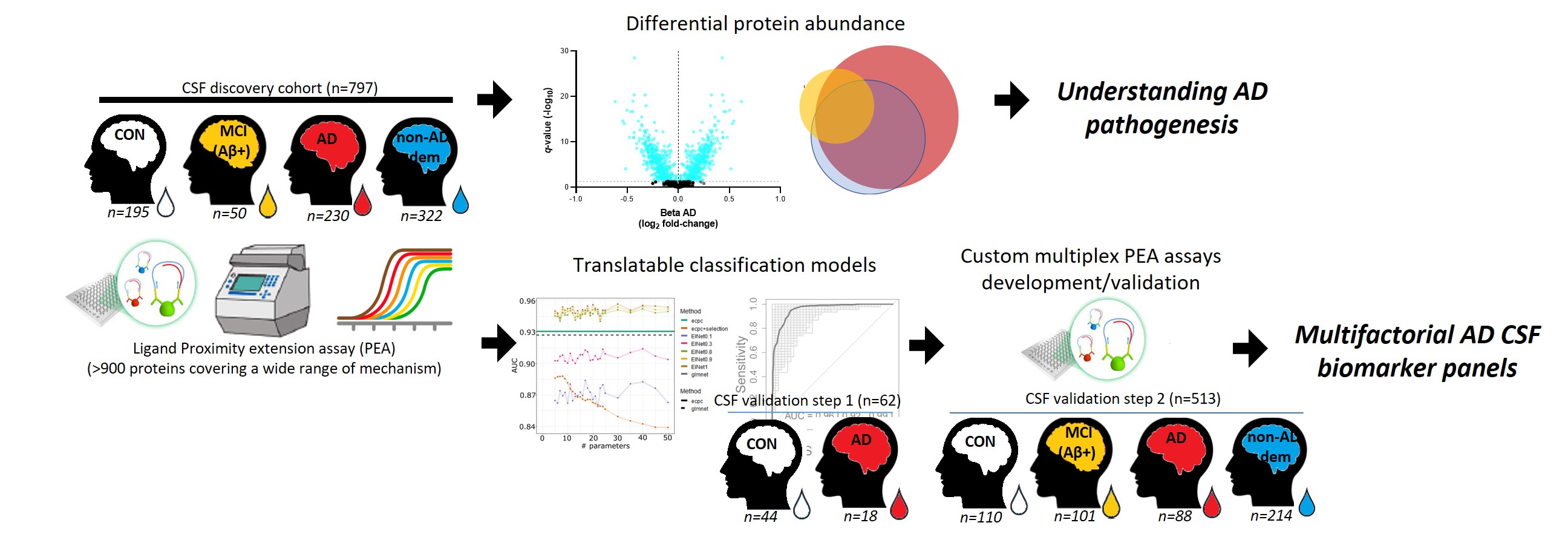 A large set of CSF proteins dysregulated along the AD continuum reflects the multifactorial nature of the disease, some of which were used to build biomarker panels with high diagnostic accuracies.
A large set of CSF proteins dysregulated along the AD continuum reflects the multifactorial nature of the disease, some of which were used to build biomarker panels with high diagnostic accuracies.
Validation of protein panels to distinguish AD
This approach allowed the researchers to translate the findings into practicable CSF biomarker panels. They selected the minimum number of proteins needed to distinguish the patient groups while they still reflected the diversity of mechanism. They made customized assays for these panels. Using these panels, they validated the strong discrimination in independent cohorts, showcasing the effectiveness of this workflow for biomarker development. These custom panels can be used in trials and research to capture the pathological diversity of AD patients.
Read the complete publication in Nature Aging.

